Celestine, also known as Celestite, is a naturally occurring mineral composed of strontium sulfate (SrSO4).
Known for its delicate pale-blue crystals and high strontium content, Celestine plays a crucial role in several high-value industries.
From advanced glass manufacturing and military-grade pyrotechnics to pharmaceuticals and metal refining, this rare mineral serves as the main source of strontium compounds used in modern technology and science.
Celestine, also known as Celestite, is a rare sulfate mineral composed primarily of strontium sulfate (SrSO₄). Its name is derived from the Latin word “caelestis”, meaning heavenly, in reference to the sky-blue color that characterizes many of its crystal formations. With its impressive purity, high strontium content, and broad range of industrial applications, Celestine stands out as a mineral of strategic importance in global supply chains.
1. Geological Characteristics and Global Occurrence
Celestine occurs in sedimentary rock formations and is commonly found in limestones and dolomites, often in association with gypsum, anhydrite, and halite. Its crystals can be tabular, prismatic, or even fibrous in texture. The most visually appealing form is translucent pale-blue crystal clusters, which are highly sought after by collectors.
Major global reserves of Celestine are found in:
-
Iran
-
Mexico
-
Spain
-
Tunisia
-
China
-
United States (Ohio, Texas, and California)
Iran is particularly known for high-grade Celestine ores with low impurities and high SrSO₄ purity, making it attractive for industrial-grade strontium production.
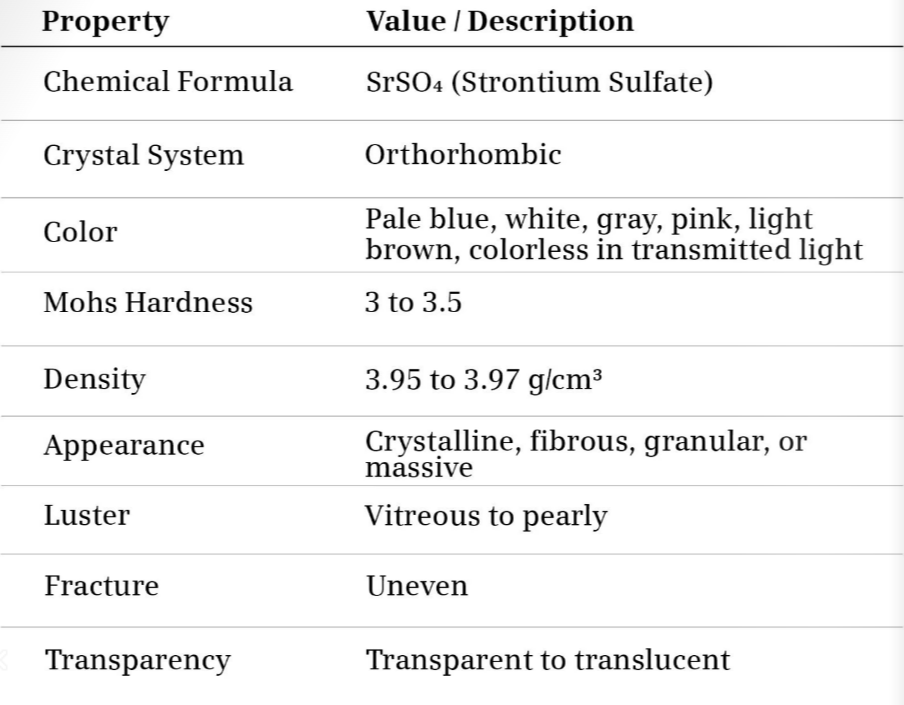
2. Chemical and Physical Properties
| Property | Description |
|---|---|
| Chemical Formula | SrSO₄ |
| Molecular Weight | 183.68 g/mol |
| Crystal System | Orthorhombic |
| Hardness (Mohs) | 3 – 3.5 |
| Specific Gravity | 3.9 – 4.0 |
| Color | Colorless, white, pale blue, grey |
| Transparency | Transparent to translucent |
| Solubility | Insoluble in water |
Celestine is thermally stable, chemically inert in many reactions, and non-toxic, which adds to its versatility in sensitive industrial environments.
3. Primary Industrial Applications
Celestine is primarily used as the raw material for strontium production, which is then converted into various strontium compounds used across multiple industries:
a. Strontium Carbonate Production
Strontium carbonate (SrCO₃) is the most commonly produced strontium compound. It is derived from Celestine via a two-step chemical process involving reduction and carbonate precipitation.
Applications include:
-
Glass manufacturing (especially cathode ray tubes and TV panels)
-
Ceramic glazes and enamels
-
Ferrite magnets
-
Pyrotechnics (as a red colorant)
b. Advanced Glass and Ceramics
Strontium-containing glass exhibits high thermal resistance, low expansion, and superior clarity. Celestine-based strontium is used in:
-
TV and CRT glass to block X-ray emissions
-
Optical lenses and heat-resistant borosilicate glass
-
Specialty ceramics used in aerospace and defense
c. Pyrotechnics and Signal Flares
Strontium salts derived from Celestine are essential in producing deep red flames in fireworks and military signal flares. Strontium nitrate and strontium chlorate offer stable combustion and vibrant coloration.
d. Pharmaceuticals and Medicine
Certain strontium compounds (e.g., strontium ranelate) have shown promise in osteoporosis treatment, enhancing bone density and reducing fracture risk.
Though not directly consumed, high-purity Celestine is essential for the pharmaceutical-grade synthesis of such compounds.
e. Metallurgy and Electrochemistry
Strontium metal is used as an alloying agent in refining aluminum and zinc, improving their mechanical properties. Celestine’s strontium is also applied in:
-
Desulfurization of molten steel
-
Production of electrodes for battery cells
-
Soldering fluxes in electronics
4. Emerging Technologies and New Uses
In recent years, strontium compounds derived from Celestine have gained traction in cutting-edge technologies, including:
-
Photoelectric sensors
-
Piezoelectric ceramics
-
Strontium titanate in microelectronics
-
Quantum computing and superconductors (experimental research)
As demand for high-performance materials grows, Celestine’s strategic role in clean energy, advanced electronics, and defense applications is expected to expand.
5. Global Market Trends
Demand Outlook:
The global market for strontium compounds is projected to grow steadily due to:
-
Increased demand in the automotive electronics industry
-
Growth in fireworks and pyrotechnic displays
-
Development of high-performance ceramics
-
Expansion of the glass and optical material sectors
Key Importers:
-
Germany
-
India
-
Japan
-
United States
-
South Korea
Many countries lack sufficient domestic Celestine deposits and rely on imports, especially from Iran, Mexico, and China, making these suppliers key players in the global trade.
6. Advantages of Iranian Celestine for Export
Iran’s Celestine reserves are among the purest in the world, with several advantages:
-
High SrSO₄ purity (often >95%)
-
Low levels of heavy metal contamination
-
Abundant, accessible surface deposits
-
Proximity to major shipping routes in the Persian Gulf
These factors make Iranian Celestine highly competitive in international markets for industrial-grade strontium production.
7. Packaging and Export Specifications
For global customers, Celestine is typically available in:
-
Lump ore (10–100 mm)
-
Crushed or milled powder (<200 mesh)
-
Bulk bags (1 MT big bags) or 25–50 kg sacks
-
Container or bulk shipment
Exporters often provide customized sizing, certificates of analysis (COA), and MSDS upon request, ensuring compliance with client-specific standards.
8. Safety and Handling
While Celestine is generally non-toxic, fine dust from crushing operations should be handled with care. Safety guidelines include:
-
Use of dust masks or respirators
-
Avoid inhalation and prolonged skin contact
-
Ensure good ventilation during processing
-
Storage in dry, covered conditions
9. Sustainability and Environmental Impact
Compared to other industrial minerals, Celestine mining has a low environmental footprint, especially when:
-
Mined from open-pit or shallow deposits
-
Processed using low-chemical-impact methods
-
Managed under responsible waste disposal practices
There is also growing interest in recycling strontium compounds from glass and ceramics to reduce dependency on virgin mineral sources.
10. Why Choose Our Celestine?
Our company offers premium-quality Celestine tailored to meet the demands of global industries. We ensure:
-
Consistent purity levels backed by laboratory reports
-
Custom packaging and grading
-
Reliable export logistics with worldwide delivery
-
Competitive pricing for bulk orders
-
Regulatory compliance and documentation for all shipments
Whether you’re a glass manufacturer in Europe, a pyrotechnics supplier in Asia, or a ceramics company in the Middle East, our Celestine products will meet your needs with quality and reliability.
Conclusion
Celestine (SrSO₄) is far more than just a beautiful mineral—it is a strategic industrial resource that supports critical technologies across the globe. From defense and electronics to health and energy, its applications are vast, and its market potential continues to grow. By sourcing high-grade Celestine from reliable suppliers, businesses can ensure a stable supply of strontium compounds for both current and future innovations.